Explain the Purposes of Blinding and Randomization in Clinical Trials
TOPICS TO BE DISCUSSEDClinical Trials Definitions Importance of trials Role of Clinical trials in Clinical Product Development Different types of Clinical trials and their phasesImportant Regulations and Guidelines ICH GCPDesign of a Clinical TrialRandomization and BlindingData Management in. Recent trials for other agents for CIDP treatment have not proved as promising with a large study of methotrexate failing to show significant benefit.

Randomization Blinding In Clinical Research Trials Castor
Randomized trials are needed to examine the effects of treating VAT on clinical outcomes since the existing randomized trials have serious limitations.

. Such trials should use a concise definition that precludes overlap with VAP or alternatively combines the diagnosis of VAT and VAP and adjusts for severity of respiratory illness. Research plans and milestones for the clinical study SBIR Phase II should be included in the PHS Human Subjects and Clinical Trials Information form. Nigerian Journal of Clinical Practice favors registration of clinical trials and is a signatory to the Statement on publishing clinical trials in Indian biomedical journals.
Randomized controlled trial RCT. Recommendations for Interventional Trials. It is also used in Trinidad and Tobago for hypertension Lans 2006 and among Mexican Americans for the treatment of type 2 diabetes mellitus DM.
An experiment such as a clinical trial in which subjects are randomly assigned to receive an experimental intervention or a control. Additional Instructions for Training. A large clinical trial showed short and long-term efficacy of IVIG for the treatment of CIDP and the US.
Behavioral trials evaluate or compare ways to promote behavioral changes designed to improve health. For example in the context of a large trial run by an experienced clinical trials unit for regulatory purposes if specific information about the randomization methods is absent it may still be reasonable to respond Probably yes rather than No information to the signalling question about allocation sequence concealment. Explain why randomization is used.
Prospective randomized clinical trials cohort studies reviews systematic reviews and meta-analysis were retrieved. Define randomization in the context of a clinical trial and give examples of appropriate methods of randomization. Food and Drug Administration approved the use of IVIG Gamunex as a treatment for CIDP.
High quality protocols facilitate proper conduct reporting and external review of clinical trials. Define blinding and explain the purpose of blinding. A process for randomly assigning subjects to different treatment groups in a clinical trial or other biomedical experiment.
Post award you will submit Study. Explain how to determine whether randomization has been successful. It is used in traditional Indian medicine for constipation colic skin diseases worm infestation and infections Heber 2007.
Integrated Addendum to E6R1 ICH Topic E6R2 in the. Clinical trials have different purposes. K12 and D43 applicants.
A total of 130 articles were finally selected for this review including 17 randomized clinical trials and 4 meta-analyses. In classical designs even though randomization and blinding techniques do not guarantee the complete elimination of unknown confounders they reduce the chance of bias and dissimilarity among the arms of a clinical trial. What that purpose is helps define the type of trial it is.
Studies on the treatment of poor obstetric outcomes in women with OAPS were included. However the completeness of trial protocols is often inadequate. Follow the instructions in your FOA.
To help improve the content and quality of protocols an international group of stakeholders developed the SPIRIT 2013 Statement Standard Protocol Items. If you are proposing any human subject studies in your application then at the time of application you must use the PHS Human Subjects and Clinical Trials Information form to submit delayed onset studiesDo not fill in Study Records. As discussed in Resources of HCs section there may be potential biases in using HCs that have to be accounted for.
Diagnostic trials study or compare tests or procedures for diagnosing a particular disease or condition. Briefly explain the differences among phase I II III IV clinical trials. One point is added for a yes answer to each of the first five items and one point is subtracted for a yes answer to either of the last.
Aloe vera has a long history of popular and traditional use. Nigerian Journal of Clinical Practice would publish clinical trials that have been registered with a clinical trial registry that allows free online access to public. Studies are scored according to the presence of three key methodological features of clinical trials specifically randomization masking and accountability of all patients including withdrawals.
This guide will help anyone who is involved in the conduct of clinical trials of drugs in human subjects in Canada to comply with Part C Division 5 of the Food and Drug Regulations the Regulations and to understand the International Council for Harmonisation ICH Guidance Document. Blinding randomization power analysis for sample size and independent replication should be included in the application wherever possible. Prevention trials look for better ways to prevent a disease in people who.

On Biostatistics And Clinical Trials Randomization Re Randomization And Micro Randomization
Evolution Of The Importance Of Randomized Clinical Trials Rct Download Scientific Diagram

Biostatistics Services Is Important For Collecting Reviewing Presenting And Interpreting Data In Clinical Research Applic Clinical Trials Clinic Trials
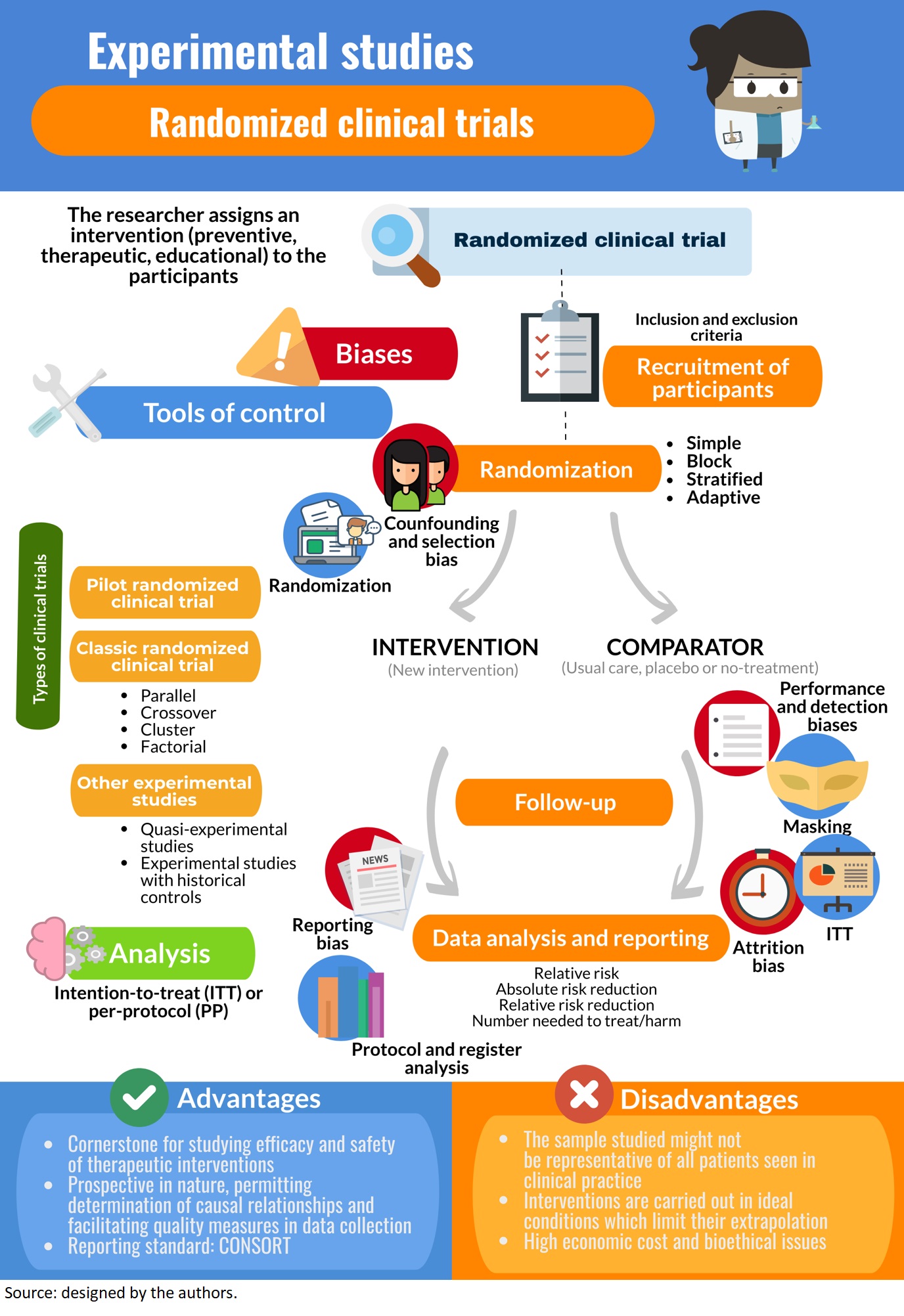
General Concepts In Biostatistics And Clinical Epidemiology Experimental Studies With Randomized Clinical Trial Design Medwave

Randomization Blinding In Clinical Research Trials Castor

Blinding Masking In Clinical Trials Youtube

Randomization In Clinical Trials Youtube

Pragmatic Randomized Clinical Trials A Proposal To Enhance Evaluation Of New Cancer Therapies With Early Signs Of Exceptional Activity Annals Of Oncology

How Clinical Trials Work Pfizer Clinical Trials Clinic Clinical Research
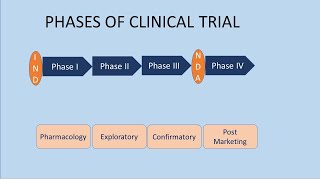
Phases Of Clinical Trials Youtube

Operationalizing Good Clinical Practice Principles In Pediatric Clinical Research The Journal Of Pediatrics

What To Report In Randomized Clinical Trials To Control Selection Bias Download Table

Pin By Aditi Joshi On 2022 Clinical Trials Clinical Research Clinic

What Is A Blinded Clinical Trial And Can It Give You Better Outcomes Aranz Medical

Randomization In Clinical Trials Qps Missouri

Descriptors Of Blinding Masking In Clinical Trial Registries Other Than Download Scientific Diagram


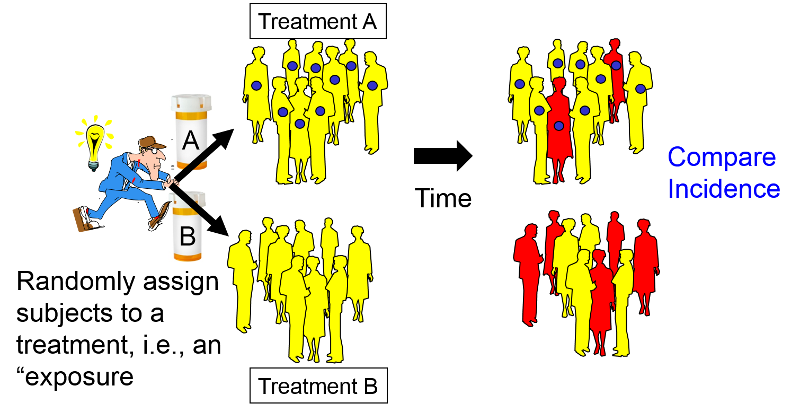
Comments
Post a Comment